Dr. Sekhar Reddy’s Developmental Biology and Basic Research Lab
Dr. Sekhar Reddy
The Protooncogene Signaling in Health and Disease research lab is led by Sekhar Reddy, PhD, who is Section Chief of Developmental Biology & Basic Research. These studies of Dr. Reddy and his collaborators focus on providing new insights into the complex physiology and pathology of childhood and adult diseases, some of which originate in the newborn. Specifically, they investigate the roles and mechanisms of endogenous transcription factors regulating oxidative stress and inflammation during alveolar remodeling and pathogenesis after neonatal and adult lung injury and their targeting potential. This research is primarily supported by the NIH.
Lab Highlights Heading link
Targeting Oxidative Stress and Protooncogone Signaling in BPD
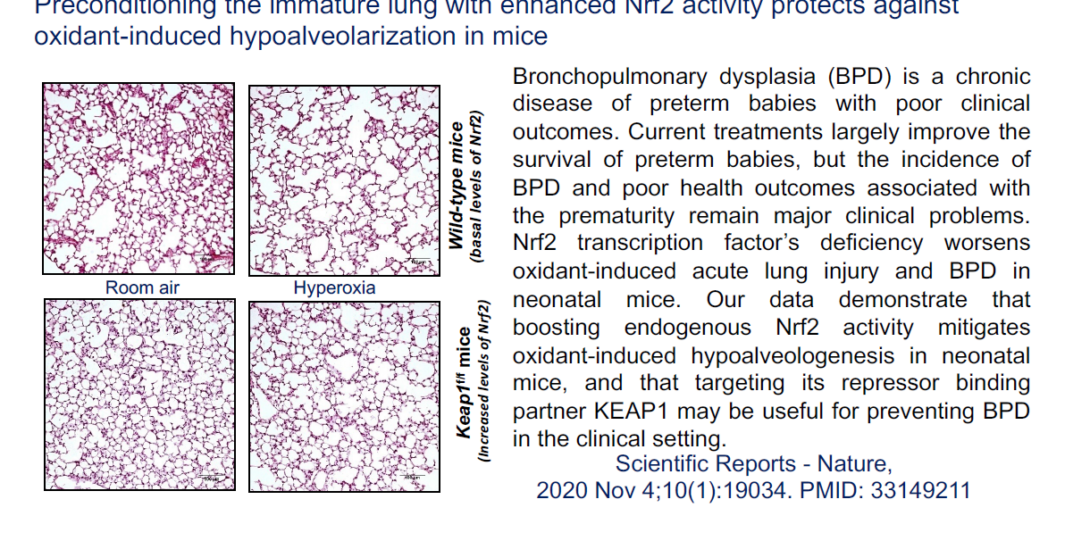
Prematurity is linked to the development of bronchopulmonary dysplasia (BPD), a chronic lung disease of preterm babies with poor health outcomes. Using a preclinical model, we defined that Nrf2-deficiency worsens and AP-1 deficiency protects oxidant-stress-induced alveolar remodeling mimicking BPD. However, preconditioning the lung with increased expression levels of Nrf2 and its target genes prevented oxidant-induced experimental BPD (Scientific Reports 2020, PMID: 33149211). Therefore, we use novel transgenic models to define and develop therapeutic strategies (small molecule activators/inhibitors) targeting proximate endogenous proteins to prevent BPD incidence in the clinical setting.
Defining Mechanisms of Inflammation Resolution and Alveolar Repair after Acute Lung Injury
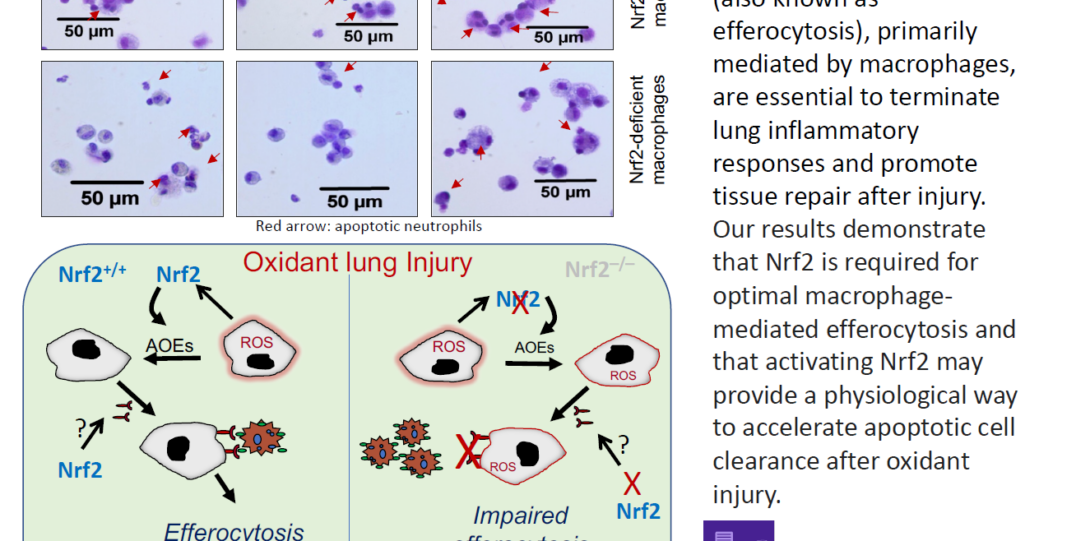
Clearance of apoptotic cells by professional phagocytes (known as efferocytosis) is crucial for homeostasis. An inefficient or dysregulated efferocytosis can impair/prolong inflammation resolution and tissue repair leading to acute lung injury (ALI/ARDS) (Faculty 1000, PMID: 33977286). Our studies (Antioxidants 2022, PMID: 35204093) demonstrated that Nrf2-dependent signaling is crucial for optimal efferocytosis, suggesting that targeting this transcription factor could be useful to accelerate dead cell clearance leading to homeostasis.
MAP Kinase Signaling in Inflammatory Lung Injury and Resolution
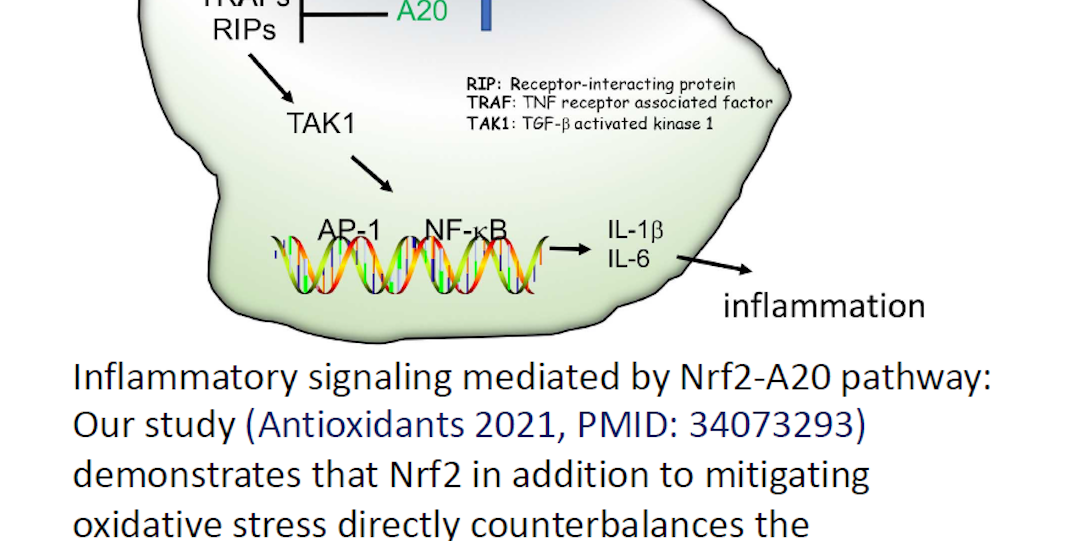
We also investigate inflammatory signaling mediated by Nrf2-A20 pathway. Our study (Antioxidants 2021, PMID: 34073293) demonstrates that Nrf2 in addition to mitigating oxidative stress directly counterbalances the unwarranted cytokine transcriptional response to mitigate amplified inflammation and maintain homeostasis. We showed that expression regulation of A20 deubiquitinase — with a dysfunction or haplo-insufficiency that leads to inflammatory disorders in mice and humans — is regulated by Nrf2.
Redox Signaling in Kidney Injury and Repair
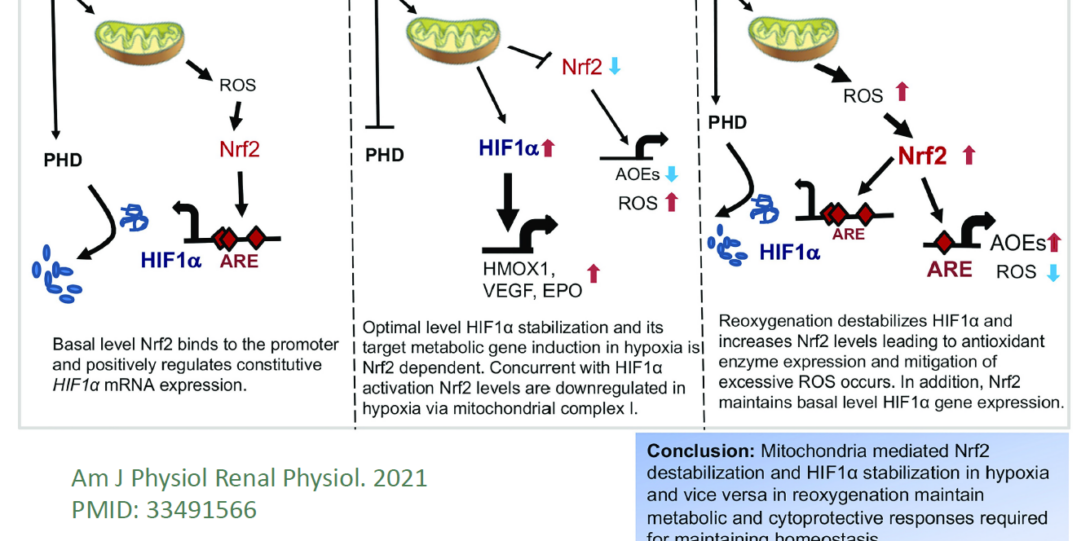
Our studies demonstrated that cytoprotective functions of Nrf2 are crucial to limiting ischemic renal reperfusion and renal toxin-induced acute kidney injury (AKI) and pathologies. Nrf2 deficiency worsens AKI, and prophylactic pharmacologic activation of Nrf2 attenuates the injury. In addition, we demonstrated that Nrf2 is required for optimal HIF1-dependent metabolic gene expression and HIF1 expression regulation by Nrf2 after hypoxia (Am J Physiol Renal Physiol. 2021 PMID: 33491566). In collaborative studies with Dr. Hamid Rabb (at Johns Hopkins), we investigate pathogenic mechanisms underlying abnormal remodeling after AKI using preclinical models and clinical samples.